Despite court orders, Tamil Nadu is yet to roll out life-saving ERT
Nine-year-old Praveen (name changed), suffering from a rare disorder, is awaiting the State government’s nod to avail of Enzyme Replacement Therapy (ERT). His mother is worried because he is growing weaker. Only treatment can help prevent more complications.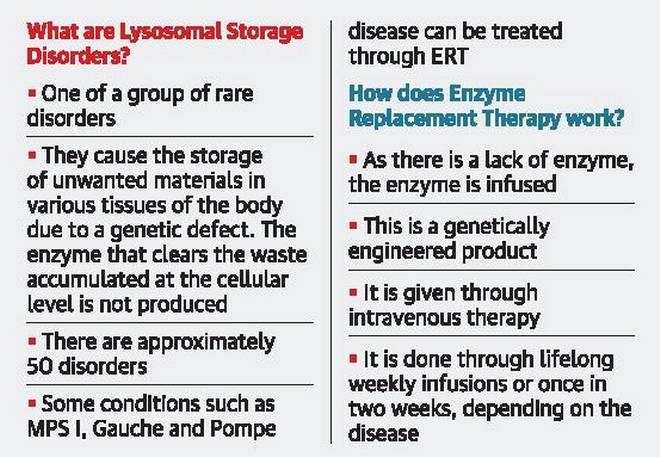
“It was when he was a two-and-a-half-year-old that he was diagnosed with mucopolysaccharidosis I. He has difficulty walking. He goes to school but he cannot exert much and cannot write. Only ERT can help in preventing his condition from worsening. He was evaluated and selected for treatment. But we are still awaiting the government’s response on providing ERT for a group of children with rare disorders, including my son,” said Pavithra (name changed), Praveen’s mother.
Risky wait
The State Technical Committee has sent a list of 27 patients with Lysosomal Storage Disorders (LSD) to the government for approval.
But the wait is risky for children with LSDs, a group of rare disorders, say parents and members of LSD Support Society (LSDSS).
“In the last one year, four children in Tamil Nadu have died waiting for treatment. We have informed the government about the deaths. ERT is life-saving medicine for children with LSDs,” said M. Raja, joint secretary and State coordinator of LSDSS, Tamil Nadu. Eighteen months ago, LSDSS filed a PIL in the Madras High Court.
The court directed the State and Institute of Child Health (ICH) to provide treatment without delay and form a committee involving all stakeholders, recalled Manjit Singh, national president of LSDSS.
“The committee was formed and the first meeting was held within two months of the judgment. The children were evaluated at ICH and a report was sent to the Health Department. But treatment has not started till date,” he said.
As of now, 18 children are getting free treatment through international charitable support, he added.
Urgent hearing
“Earlier this month, we made an application to the Registrar General of the Madras High Court for an urgent hearing. We are appealing to the government to start treatment for a handful of children who urgently need ERT,” he said.
Sujatha Jagadeesh, head, Department of Genetics, MediScan, said as of now, 28 children are on treatment at the centre, while another 15 to 20 are on the waiting list. “It is the prerogative of the State to collect data on rare disorders. “Once data is pooled, we will know the disease burden… The government can have an agreement with the drug manufacturers to provide medicines,” she said. An official said the State government was waiting for the Centre to revise guidelines in the National Policy for Treatment of Rare Diseases. “In the meantime, ICH has started to provide supportive care for these children,” he said.



