 We must applaud ourselves. We must applaud ourselves because we have toiled and advocated the causes of affordable healthcare and access to drugs. We have fought for cancer and malaria medication to be made available at naught a cost to the poor, ailing millions (well with cancer we tried anyway). While battle lines are yet being drawn, as we face up against Big Pharma and the corrupt, rickety governments that ensure they guzzle all our money, there is a group of people that we have failed as well.
We must applaud ourselves. We must applaud ourselves because we have toiled and advocated the causes of affordable healthcare and access to drugs. We have fought for cancer and malaria medication to be made available at naught a cost to the poor, ailing millions (well with cancer we tried anyway). While battle lines are yet being drawn, as we face up against Big Pharma and the corrupt, rickety governments that ensure they guzzle all our money, there is a group of people that we have failed as well.
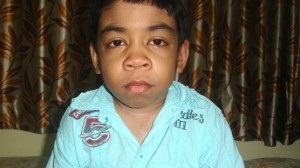
Let me introduce you to Arian. Arian is a 11 year old boy who suffers from a condition known as the Hunter syndrome. A person afflicted by this syndrome is unable to process certain substances in their body, which accumulates as a deposit on places on the body and interferes with its normal functioning. The complications resulting from Hunter syndrome are often life limiting. Arian is one among 2000 people worldwide who suffer from Hunter syndrome, earning it the characterisation of a rare disease.
What is a rare disease, you ask? A rare disease or an orphan disease is a disease that affects a very small amount of the population, often quantified to be around 0.1% of the total population. Most rare diseases are genetic, and are present throughout a person?s entire life, even if symptoms do not appear immediately. Some other rare diseases are Progeria and Asperger Syndrome both of which are rare, complex life-threatening diseases.Asperger Syndrome is a condition in which patients have difficulty interacting socially, repeat behaviours, and clumsiness.
Even as we observed World Rare Disease Day on the 28th of February, these diseases continue to pose a mammoth problem in terms of both access and cost of medication that can be used to treat these diseases. Arian needs to undergo an enzyme replacement therapy developed by Shire, a US drug company, which is unavailable in India, and so expensive (about $375,000 for a year?s treatment) that it is often carried out with government funding in countries like the UK, Germany and in parts of Canada.
Why is this such a problem, you ask? Pharmaceutical companies, like most businesses operate tend towards markets where there is a larger possibility to sell a product. By virtue of their very definition, rare diseases do not offer such a market, wherein the companies can realise the profits that they expected to make on the sale of a particular drug. Therefore, it was considered to be more profitable if they focussed their efforts into developing cures for more common diseases, or more accurately, developing treatments which are more likely to give them profits. There is another disturbing pattern with regards to rare diseases. That they are unevenly spread across the world, with developing countries who have a higher number of patients, and who can?t afford these drugs, suffering from these diseases. For instance, in the United States, the number of people affected by rare diseases is 25 to 30 million, with a similar number in the whole of Europe. India, on the other hand has about 79 million patients suffering from rare diseases. Also, to be noted is that there are over 6000 different rare diseases and the market on a drug developed specifically for each of these is going to be therefore proportionately smaller. For instance, there are only 2000 people worldwide who suffer from Hunter syndrome, constituting but a drop in the ocean. The argument is therefore that there isn?t enough incentive to innovate in this regard. But we must ask ourselves the tough questions here. Are these 79 million people in India not to get adequate medical attention? Are we as a society going to allow profit motives to stand in the way of essential innovation?
But is there a solution, you ask? In drawing the fine line between having adequate incentives to innovate and the patient needs worldwide, there are compromises to be made. Before I get into that aspect however, there is a recent trend that allows for a glimmer of optimism. The British
https://premier-pharmacy.com/product-category/adhd/
Medical Journal in an article, notes that there are players other than Big Pharma in the market who do in fact develop drugs (orphan drugs they are called) for these rare diseases ? and these players include small and medium size research centres and public institutions like universities. The article notes that nearly 72% of orphan drugs originated from these sources. Coming to the compromises that the Law can allow, the situation in the United States, is an interesting case study. The Orphan Drug Act (ODA) passed in 1983, grants various incentives for the development of drugs including a seven-year market exclusivity for companies that developed orphan drugs, grants for drug development, tax credits equal to nearly 50 percent of clinical trial costs, fast-track approvals of drugs indicated for rare diseases among others. The interesting thing to note here is the market exclusivity rights and the tax credits. The Act ensures that no other similar molecule can be marketed for a period of 7 years. The similarity test here used is one of clinical similarity and not one of structural similarity between the molecules. This is important because most rare diseases are genetic and the cures to these diseases are often biological rather than chemical, so it?s often not possible to apply the structural similarity test that easily. This also ensures an advantage for the patients themselves as well as for innovators because this ensures that a company has exclusivity in the market which is immune from mere re-engineering of the molecule by making structural changes in that molecule, ensuring that there is an incentive to innovate (read our post here). The European Union also provides similar incentives for orphan drug makers, extending the exclusivity period to 10 years instead of 7.
The Indian effort has not been so heartening. The government is barely involved in the promotion of awareness about these diseases let alone any other work in this direction. It is only organisations like the Organisation for Rare Diseases in India and a couple of other NGOs that are active in the field. There is no legislation in India to promote orphan drug development.
The patent regime itself does not inherently cater to the interests of patients who are in the minority (orphan disease patients). Companies are often posed with problems such as generic manufacturing which eats away their market share. Now while generics might increase access in the case of ordinary drugs with a large patient base for sale, in the case of orphan drugs it is just going to eat away the already miniscule patient base that an innovator has to sell to, thereby drastically reducing his incentive to innovate. There are two considerations here incentivizing the innovation of a treatment and, finding ways for it to be accessible. The first issue is of more importance though and should be treated as such. It would be worth the high price since having ?a? treatment, even if expensive, is simply better than no treatment at all. Another important problem that the Indian patent regime poses to development of orphan drugs is S. 3(d). A lot of orphan drugs are often pre-existing drugs (read on page 7 here) that are discovered to have multiple functions. Now, S. 3(d) may not allow for such molecules to be granted protection for their rare disease application as it prevents the patenting of the mere discovery of any new property or new use for a known substance.
The point of this rather long and painful rant is that much more policy discussion needs to be had on this often neglected issue which hopefully might generate some reform. IP law alone does not provide an adequate solution to this tricky problem. Government efforts in terms of legislation (that grants exclusivity as well as patent protection) as well as subsidisation are the need of the hour. The United States Orphan Drug Act is definitely an example to follow, and its success is indeed heartening.
Written by Thomas J. Vallianeth
https://spicyip.com/2014/03/rare-diseases-and-innovation.html
 We must applaud ourselves. We must applaud ourselves because we have toiled and advocated the causes of affordable healthcare and access to drugs. We have fought for cancer and malaria medication to be made available at naught a cost to the poor, ailing millions (well with cancer we tried anyway). While battle lines are yet being drawn, as we face up against Big Pharma and the corrupt, rickety governments that ensure they guzzle all our money, there is a group of people that we have failed as well.
We must applaud ourselves. We must applaud ourselves because we have toiled and advocated the causes of affordable healthcare and access to drugs. We have fought for cancer and malaria medication to be made available at naught a cost to the poor, ailing millions (well with cancer we tried anyway). While battle lines are yet being drawn, as we face up against Big Pharma and the corrupt, rickety governments that ensure they guzzle all our money, there is a group of people that we have failed as well.


